Prof. Hagai Abeliovich
Major research interests:
Yeast Cell Biology and Biotechnology
Current research projects:
- Understanding the mechanism of regulation of nutrient–starvation induced autophagy in yeast and its potential implication for industrial fermentations.
- Understanding the mechanism of mitochondrial turnover through mitophagy in the yeast S. cerevisiae.
- Selective effects of weak organic acid food preservatives on intracellular membrane trafficking.
Metabolic engineering of yeast for the production of plant nutraceuticals.
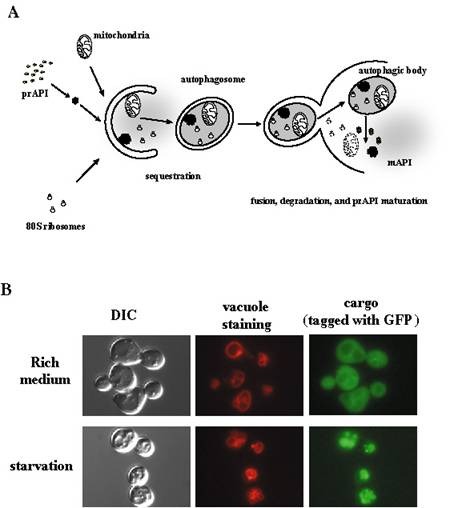
A. Overview of membrane trafficking in yeast macroautophagy.
During autophagy, various components of the cytoplasm, such as mitochondria, ribosomes, specific cargo such as prAPI (see part B of this figure), and soluble cytoplasmic material (depicted as diffuse gray) are sequestered into autophagosomes, which are large (300–900 nm) double bilayer vesicles. Once formed, autophagosomes fuse with the vacuole, releasing a single–bilayer bound autophagic body into the lumen of the vacuole. Vacuolar hydrolases act upon the limiting membrane of the autophagic body, releasing its content which is degraded into biosynthetic building blocks.
B. Fluorescent microscopy of autophagic trafficking. A specific autophagic cargo, Aut7, was fused with green fluorescent protein (GFP). In normally growing cells, GFP–Aut7 fluorescence is largely cytosolic. Upon induction of autophagy by starving the cells for nitrogen, Aut7 is delivered into the lumen of the vacuole (the yeast equivalent of the lysosome). Top row: rich medium; Bottom row: starvation. Left panels, bright field (DIC); middle panels, vacuole membrane (stained by a specific red fluorescent dye); right panels, GFP–Aut7. One can observe that the GFP fluorescence coincides with the vacuolar staining in the starved cells (bottom row), but not in the cells that are growing normally (top row).